Abstract
Introduction: Plamotamab is a humanized bispecific antibody that binds both CD20 and CD3 and recruits cytotoxic T cells to kill CD20-expressing malignant cells. In the dose-escalation phase of an ongoing first-in-human (FIH) Ph 1 study (XmAb13676-01; NCT02924402), plamotamab was well tolerated with manageable cytokine release syndrome (CRS) and demonstrated evidence of clinical activity in heavily pretreated patients (pts) with relapsed/refractory (R/R) non-Hodgkin's lymphoma (NHL) (Patel; ASH 2021). Here, exposure-response (ER) analyses of the dose-escalation cohorts from the same study are reported. Safety and efficacy results from the follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL) escalation (Part C) and expansion cohorts at the proposed recommended dosing regimen (RD) are also reported.
Methods: The study is an FIH, multi-center, open-label Ph 1 dose-escalation study in R/R NHL pts. The dose-escalation phase has 3 Parts: Parts A and B using weight-based dosing; Part C using flat step-up doses (SUD). An IV RD was identified from Part C, which was used to expand into FL and DLBCL. Treatment was continued for ≥2 cycles if therapeutic benefit was evident. ER analysis was conducted based on exposure metrics calculated using population pharmacokinetic (popPK) model for plamotamab vs. observed responses related to efficacy/safety from Parts A, B, and C.
Results: At data cut-off for RD safety/efficacy evaluation (25July2022), 36 pts were enrolled on or before 01April2022 (median age: 67 years, range: 36-86; 61% male). At baseline, 11.1% had stage III disease and 69.4% had stage IV disease. Median number of prior lines of therapy was 4 (range: 2-10), with 50% pts receiving CAR-T as a prior therapy (16 pts with DLBCL, 2 pts from other NHL subtypes, and no pts with FL). After last therapy, 91.7% of all pts had relapsed/progressed.
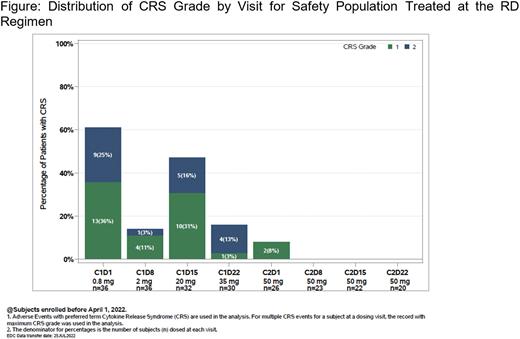
In the RD safety population (n=36), CRS was the most common adverse event (AE; 72.2%). CRS was mostly confined to Cycle 1 and no Grade (Gr)≥3 CRS was reported. Treatment-emergent AEs (≥25%) included anemia (44.4%), pyrexia and nausea (38.9% each), asthenia (27.8%), and aspartate aminotransferase increase, diarrhea, and hypophosphatemia (25.0% each). Gr≥3 AEs (≥10%; 77.8% overall) were anemia (19.4%), neutropenia and neutrophil count decreased (16.7% each) and thrombocytopenia (11.1%). SAEs (≥5%; 55.6% overall) were CRS (25.0%), COVID-19 (8.3%), pneumonia and sepsis (5.6% each). AEs leading to plamotamab discontinuation occurred in 5 pts (13.9%).
Overall, 25 pts were evaluable for efficacy at the RD. DLBCL pts at the RD (n=19) had an objective response (OR) rate of 47.4% (9/19 pts) and CMR/CR rate of 26.3% (5/19 pts); DLBCL pts with prior CAR-T had an OR rate of 46.2% (6/13 pts) and CMR/CR rate of 30.8% (4/13 pts). In FL pts at the RD (n=6), OR rate was 100% (6/6 pts) and CMR/CR rate was 50% (3/6 pts). The median time to initial response was 54 days for DLBCL and 56.5 days for FL.
At data cut-off for ER analysis (10Nov2021) of Parts A, B and C only, exposure data were available from 55 pts who received at least 1 dose of plamotamab in the second cycle, were evaluable for CRS and for efficacy and safety at the target dose. For the first "priming” dose, plamotamab exposure correlated significantly with peak IL6 levels as well as incidence of Gr≥2 CRS events. At subsequent SUD, Postdose-Cmax / Predose-Ctrough ratio, indicative of the fold-change with each SUD, was the best predictor of any grade CRS events. At the target dose, no significant relationship exists between plamotamab exposure vs. all Gr≥3 AEs. A positive relationship exists for best OR, which is further improved by accounting for the impact of residual rituximab on plamotamab binding to CD20 using a competitive binding model.
Conclusion: Plamotamab demonstrated evidence of clinical activity in heavily pretreated pts with DLBCL and FL with promising responses in pts with prior CAR-T therapy. CRS was generally manageable with premedication. Safety and efficacy evaluation of pts at the RD is currently ongoing, and updated results will be provided. Taken together, popPK/PD analysis of plamotamab exposure with IL6 levels, CRS incidence, high-grade AEs and OR rate provide guidance for RDs to be studied in future trials. A novel exposure metric was proposed to inform step-up dose increases that minimize the risk of CRS, while impact of rituximab on drug efficacy was captured as has been described in the literature.
Disclosures
Patel:Curis, Inc: Research Funding; Epizyme: Consultancy, Research Funding; MEI Pharma: Consultancy, Research Funding; Abbvie: Consultancy; Adaptive Biotechnologies: Research Funding; Fate Therapeutics: Research Funding; Kite pharma: Consultancy, Research Funding, Speakers Bureau; Genetech/Roche: Consultancy, Research Funding, Speakers Bureau; Sunesis Pharmaceuticals: Research Funding; Velos Bio: Research Funding; Pharmacyclics/Janssen: Consultancy, Research Funding, Speakers Bureau; ADC Therapeutics: Consultancy; Aptevo Therapeutics: Research Funding; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; BeiGene: Consultancy; Bristol Myers Squibb: Consultancy, Research Funding, Speakers Bureau; Caribou Biosciences: Consultancy; Celgene: Consultancy, Research Funding, Speakers Bureau; CRISPR Therapeutics: Research Funding; Morphosys: Consultancy; Nurix: Research Funding; Xencor: Consultancy, Research Funding; Loxo Oncology: Consultancy, Research Funding; TG Therapeutics: Consultancy, Speakers Bureau; Trillium Therapuetics/Pfizer: Consultancy, Research Funding. Riedell:Fate Therapeutics: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees; Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; MorphoSys: Research Funding; Tessa Therapeutics: Research Funding; Xencor: Research Funding; Calibr: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Intellia Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sana Biotechnology: Consultancy; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Tilly:Incyte: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding. Ahmed:Tessa Therapeutics: Consultancy, Research Funding; Chimagen: Consultancy, Research Funding; Myeloid Therapeutics: Consultancy; Servier: Membership on an entity's Board of Directors or advisory committees; Seagen: Research Funding; Merck: Research Funding; Xencor: Research Funding. Michot:Sanofi: Research Funding; Roche: Other: Nonfinancial support, Research Funding; NH TherAGUuiX: Other: Nonfinancial support; Pfizer: Other: Nonfinancial support, Research Funding; Merck: Other: Nonfinancial support, Research Funding; Janssen Cilag: Research Funding; INCa: Research Funding; MedImmune: Other: Nonfinancial support; GlaxoSmithKline: Other: Nonfinancial support, Research Funding; Boehringer Ingelheim: Other: Nonfinancial support, Research Funding; Bristol Myers Squibb: Other: Nonfinancial support, support for travel to and accomodation at EHA 2022 to present CC-99282-NHL-001 study data;, Research Funding; AstraZeneca: Other: Nonfinancial support, Research Funding. Ghesquieres:Gilead: Consultancy, Honoraria; BMS: Honoraria; Roche: Consultancy, Honoraria; Abbvie: Honoraria. Schiano de Collela:Abbvie: Honoraria; Takeda: Honoraria; Janssen: Honoraria. Bouabdallah:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy, Honoraria; Abbvie: Honoraria; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Milteny Biomedicine: Honoraria, Membership on an entity's Board of Directors or advisory committees. Iyengar:Beigene: Membership on an entity's Board of Directors or advisory committees; Lilly: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: conference support, Speakers Bureau; AbbVie: Other: conference support; Janssen: Speakers Bureau; Kite Gilead: Membership on an entity's Board of Directors or advisory committees. Clynes:Xencor: Current Employment, Current equity holder in publicly-traded company. Kanodia:Xencor: Current Employment, Current equity holder in publicly-traded company. Bao:Xencor: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Ding:Xencor: Current Employment. Jin:Xencor: Current Employment, Current equity holder in publicly-traded company. Ainsworth:AbbVie Inc.: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Xencor: Current Employment, Current equity holder in publicly-traded company. Garcha:Xencor: Current Employment, Current equity holder in private company. Kye:Xencor: Current Employment, Current equity holder in publicly-traded company. Phillips:Epizyme: Consultancy; Gilead: Consultancy; Genmab: Consultancy; Bayer: Consultancy; Genentech: Consultancy, Research Funding; Eli Lilly: Consultancy; Beigene: Consultancy; ADC Therapeutics: Consultancy; Xencor: Consultancy; AbbVie: Consultancy, Research Funding; Pharmacyclics: Consultancy; Incyte: Consultancy, Other: Travel Expenses ; AstraZeneca: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal